Product Description
What is Choline Glycerophosphate?
Choline Glycerophosphate is a compound containing choline. It is not produced by direct extraction but through an esterification and neutralization reaction, and purified, filtered, and dried to achieve a regular composition and very high purity suitable for commercial use. The end product typically comes in the form of a white to off-white crystalline or fine powder, in a stable form, which allows it to be weighed, mixed, and processed on a large scale. Its main active ingredient is choline glycerophosphate, a molecular structure that fuses choline and glycerophosphate to provide a major source of choline in a form that is worth considering in the reliability of formulations. In terms of functionality, it does not require any special preparation but is used directly as a functional nutrient ingredient or formulation component, which is why it is applicable in a compliant application and is based on material performance and nutritional composition. It is used extensively in the dietary supplement manufacturing industry, functional food and beverage development industry, supplying nutraceutical ingredients, and special animal nutrition industries, where it is a standardized raw material readily incorporated in powders, capsules, tablets, and premix systems.
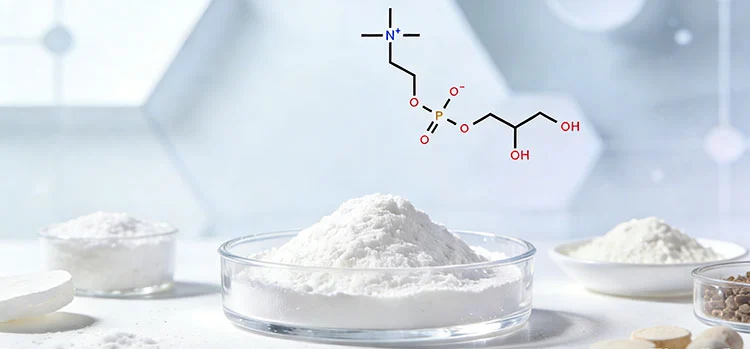
Analysis
ANALYSIS | SPECIFICATION | RESULTS |
Appearance | White powder | Complies |
Odor | Characteristic | Complies |
Tasted | Characteristic | Complies |
Assay | 98% | Complies |
Sieve Analysis | 100% pass 80 mesh | Complies |
Loss on Drying | 5% Max. | 1.02% |
Sulphated Ash | 5% Max. | 1.30% |
Extract Solvent | Ethanol & Water | Complies |
Heavy Metal | 5ppm Max | Complies |
As | 2ppm Max | Complies |
Residual Solvents | 0.05% Max. | Negative |
Microbiology | ||
Total Plate Count | 1000/g Max | Complies |
Yeast & Mold | 100/g Max | Complies |
E.Coli | Negative | Complies |
Salmonella | Negative | Complies |
Benefits
1. Defined Source of Choline
Choline Glycerophosphate is a well-defined choline-based ingredient and enables manufacturers to develop products that have regulated choline input and managed specification transparently.
2. High Formulation Compatibility
Its chemical composition allows it to be easily combined with a large variety of diverse common ingredients, and allows its stable integration into powders, capsules, tablets, and premix systems without disrupting the total integrity of the formulation.
3. Process and Handling Stability
The ingredient also shows stable physical characteristics through the regular industrial steps like blending, filling, and packaging, which facilitates a reliable performance of the ingredient in large scale production setting.
4. Support for Precise Standardization
The usefulness is that of a constant assay and a specified composition that assists manufacturers in ensuring consistency from batch to batch, and in ensuring quality control standards within their own parameters.
5. Versatile Application Potential
Its neutral material properties allow it to be utilized in a variety of product categories and formulation ideas, and offer it some flexibility to develop products and expand the product portfolio.
6. Alignment with Modern Ingredient Trends
The compound fits the existing industry trends towards traceable, well-documented, and chemically stable components, which assist in long term planning and market positioning that are compliant.

Application
1. Dietary Supplement Manufacturing
It is an ingredient used in the manufacturing of capsules, tablets, powders, and granules in which a uniform composition and predictable processing behavior are needed.
2. Functional Food and Beverage Development
The ingredient has found application in functional food systems like nutrition powders, fortified mixes, and beverage premixes, which facilitate homogenous blending and uniform final product profiles.
3. Nutraceutical Ingredient Supply Chains
Being a bulk nutraceutical input, it is also provided to formulation companies and brand owners that require well-documented specifications, traceability, and scalable sourcing solutions.
4. Animal Nutrition and Feed Formulation
Choline Glycerophosphate finds use in animal feed and premix systems, being chosen due to its ability to be compatible with normal feed processing and its predictable material properties.
5. Contract Manufacturing and Private Label Services
Contract manufacturers and OEM/ODM service providers utilize the compound greatly because of its formulation flexibility and ability to custom-made specifications.
6. Research, Development, and Formulation Testing
It is also applied in the research and development settings in formulation optimization, stability testing, and ingredient benchmarking in compliant product development systems.
The Best Choline Glycerophosphate Supplier
Having over 15 years of committed work experience in ingredient production and international distribution, the company offers it as a highly reliable raw material to use in professional formulation and large quantity production. We emphasize our business operations on controlled production management, constant quality parameters, and clear technical documentation as the means of supporting the long-term cooperation with international partners. We believe in customized OEM /ODM services where specifications can be customized to suit various market and application needs, including concentration range, physical form, packaging, and even labelling as a personal label. With a background in industry and flexible manufacturing potential and responsive project management, we assist our customers to simplify sourcing, enhance the efficiency of formulation, andensure a stable supply.
Why Choose Us?
Our quality assurance is based on a strict choice of raw materials, accuracy in process control, and inspection procedures that guarantee the consistent and traceable Choline Glycerophosphate for industrial application. Our pricing process is cost-efficient and of high quality, as it provides competitive solutions in the long term procurement. At the service level, we focus on active communication, prompt order fulfillment, and real-world technical support to ensure that clients face no problems in formulating and supplying the chain-of-custody services. Through a mixture of quality assurance, transparent pricing, and responsive service, we offer a total and professional sourcing experience to businesses.
Where to Buy Choline Glycerophosphate?
In order to inquire the product specifications, prices, OEM/ODM offers, please write to us at info@scigroundbio.com and fill your needs in the below requirement form and we shall offer timely advice and a customized solution to your sourcing requirements.


Hot Tags: Choline Glycerophosphate, v), China, manufacturers, GMP factory, suppliers, factory, wholesale, best, price, buy, for sale, bulk, 100%pure.
Send Inquiry
.webp)







